- Home ·
- Publications ·
- Research Area F ·
- 2008 ·
Intramembrane Proteolysis of GxGD-type Aspartyl Proteases is slowed by a Familial Alzheimer Disease-like Mutation
03-Sep-2008
Regina Fluhrer, Akio Fukumori, Lucas Martin, Gudula Grammer, Martina Haug-Kröper, Bärbel Klier, Edith Winkler, Elisabeth Kremmer, Margaret M. Condron, David B. Teplow, Harald Steiner, Christian Haass
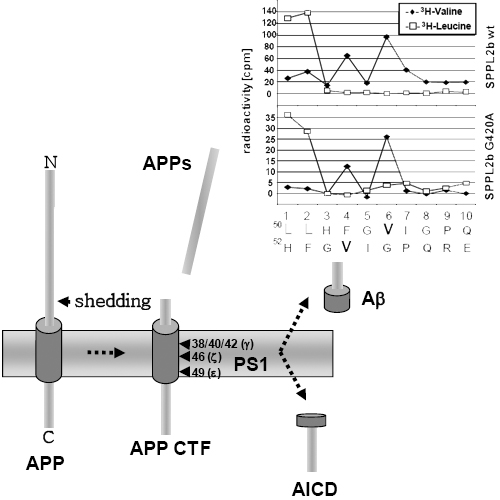
More than one hundred and fifty familial Alzheimer's Disease (FAD)-associated missense mutations in presenilins (PS1 and PS2), the catalytic subunit of the γ- secretase complex, cause aberrant Amyloid β-peptide (Aβ) production, by increasing the relative production of the highly amyloidogenic 42 amino acid variant. The molecular mechanism behind this pathological activity is unclear and different possibilities ranging from a gain of function to a loss of function have been discussed. γ-Secretase, signal peptide peptidase (SPP) and SPP-like proteases (SPPLs) belong to the same family of GxGD-type intramembrane cleaving aspartyl proteases and share several functional similarities. We have introduced the FAD-associated PS1 G384A mutation, which occurs within the highly conserved GxGD motif of PS1 right next to the catalytically critical aspartate residue, into the corresponding GxGD motif of the signal peptide peptidase-like 2b (SPPL2b). Compared to wt SPPL2b, mutant SPPL2b slowed intramembrane proteolysis of tumor necrosis factor α (TNFα) and cause a relative increase of longer intracellular cleavage products.Since the N-termini of the secreted counterparts remain unchanged, the mutation selectively affects the liberation of the intracellular processing products. In vitro experiments demonstrate that the apparent accumulation of longer intracellular cleavage products is the result of slowed sequential intramembrane cleavage. The longer cleavage products are still converted to shorter peptides, however only after prolonged incubation time. This suggests that FAD-associated PS mutation may also result in reduced intramembrane cleavage of β-Amyloid Precursor Protein (βAPP). Indeed, in vitro experiments demonstrate slowed intramembrane proteolysis by γ-secretase containing PS1 with the G384A mutation. As compared to wt PS1, the mutation selectively slowed Aβ40 production, while Aβ42 generation remained unaffected. Thus, the PS1 G384A mutation causes a selective loss of function by slowing the processing pathway leading to the benign Aβ40.