Improved deep two-photon calcium imaging in vivo
21-Dec-2016
Cell Calcium, http://dx.doi.org/10.1016/j.ceca.2016.12.005
Cell Calcium, online article
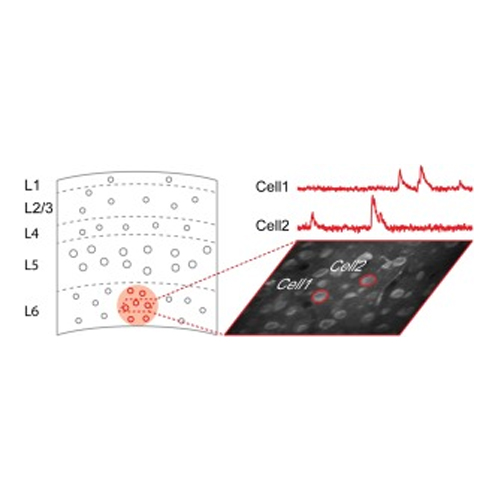
Two-photon laser scanning calcium imaging has emerged as a useful method for the exploration of neural function and structure at the cellular and subcellular level in vivo. The applications range from imaging of subcellular compartments such as dendrites, spines and axonal boutons up to the functional analysis of large neuronal or glial populations. However, the depth penetration is often limited to a few hundred micrometers, corresponding, for example, to the upper cortical layers of the mouse brain. Light scattering and aberrations originating from refractive index inhomogeneties of the tissue are the reasons for these limitations. The depth penetration of two-photon imaging can be enhanced through various approaches, such as the implementation of adaptive optics, the use of three-photon excitation and/or labeling cells with red-shifted genetically encoded fluorescent sensors. However, most of the approaches used so far require the implementation of new instrumentation and/or time consuming staining protocols. Here we present a simple approach that can be readily implemented in combination with standard two-photon microscopes. The method involves an optimized protocol for depth-restricted labeling with the red-shifted fluorescent calcium indicator Cal-590 and benefits from the use of ultra-short laser pulses. The approach allows in vivo functional imaging of neuronal populations with single cell resolution in all six layers of the mouse cortex. We demonstrate that stable recordings in deep cortical layers are not restricted to anesthetized animals but are well feasible in awake, behaving mice. We anticipate that the improved depth penetration will be beneficial for two-photon functional imaging in larger species, such as non-human primates.