Entrapment of Water at the Transmembrane Helix–Helix Interface of Quiescin Sulfhydryl Oxidase 2
19-Feb-2016
Biochemistry, 55 (9), pp 1287–1290, DOI: 10.1021/acs.biochem.5b01239
Biochemistry, online article
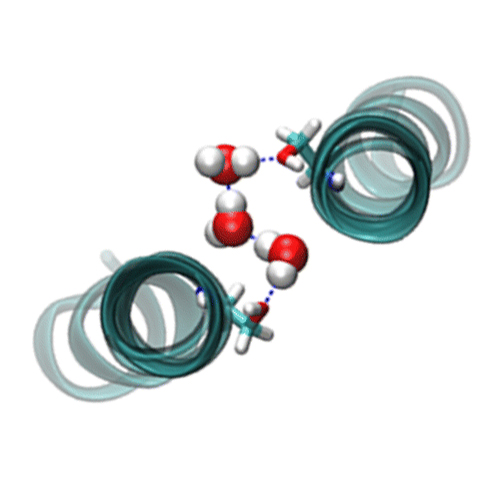
Little is known about how a membrane can regulate interactions between transmembrane helices. Here, we show that strong self-interaction of the transmembrane helix of human quiescin sulfhydryl oxidase 2 rests on a motif of conserved amino acids comprising one face of the helix. Atomistic molecular dynamics simulations suggest that water molecules enter the helix–helix interface and connect serine residues of both partner helices. In addition, an interfacial tyrosine can interact with noninterfacial water or lipid. Dimerization of this transmembrane helix might therefore be controlled by membrane properties controlling water permeation and/or by the lipid composition of the membrane.