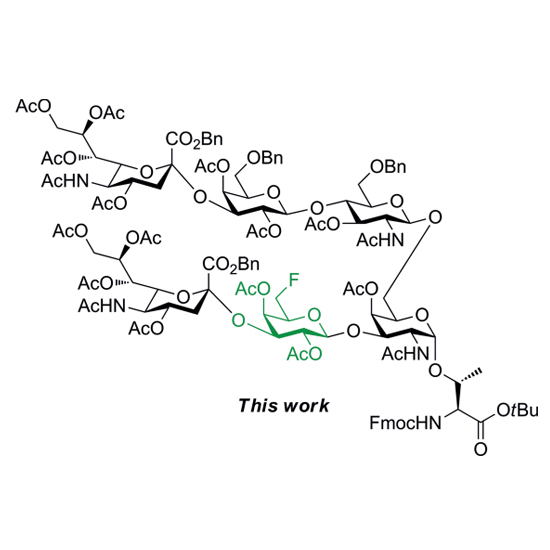
Synthesis of a Fluorinated Sialophorin Hexasaccharide–Threonine Conjugate for Fmoc Solid-Phase Glycopeptide Synthesis
27-Jun-2016
Eur. J. Org. Chem., Volume 2016, Issue 22, Pages 3709–3720, DOI: 10.1002/ejoc.201600523
Eur. J. Org. Chem., online article
The decoding of the mechanisms underlying glycan-mediated recognition in disease and health requires access to structurally well-defined oligosaccharides as molecular probes. Owing to their often favourable properties, deoxyfluorosugars have emerged as a promising class of selectively modified carbohydrates for biological and immunological studies. In particular the enhanced metabolic stability and intrinsic immunogenicity of fluorinated carbohydrates has spurred research on their use for vaccine design. Herein, a first total synthesis of an orthogonally protected and fluorinated hexasaccharide–threonine conjugate of the natural sialophorin antigen has been accomplished. Starting from readily available monosaccharide building blocks, the targeted glycosyl amino acid 1 was assembled by a [3+3′]-block glycosylation strategy. Together with its (1→4)-linked regioisomer the glycan mimic can be applied to solid-phase glycopeptide synthesis to access novel sialophorin-derived molecular tools for functional and biomedical studies.