Stereoselective Total Synthesis of Bisorbicillinoid Natural Products by Enzymatic Oxidative Dearomatization/Dimerization
03-Aug-2017
Angew. Chem. Int. Ed., Volume 56, Issue 42, Pages 12888-12891, https://doi.org/10.1002/anie.201705976
Angew. Chem. Int. Ed., online article
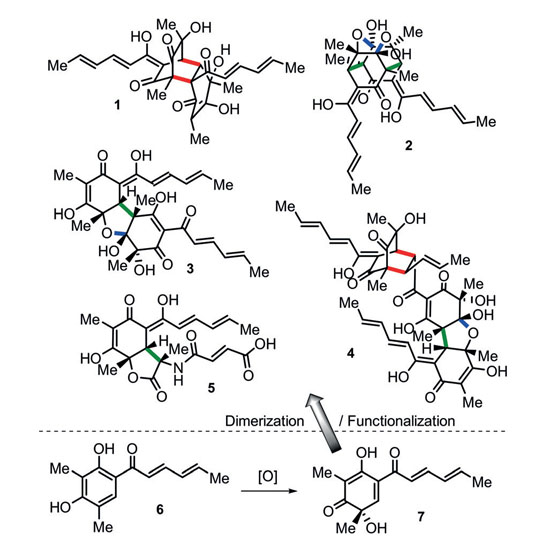
Natural products are a virtually inexhaustible source of small molecules with spectacular molecular architectures and biomedical potential. Their structural complexity generates formidable challenges to total synthesis but often also precludes time‐ and resource‐efficient, stereoselective synthetic access. Biosynthetically, nature frequently uses dimerization and oligomerization reactions to produce highly challenging frameworks from simple starting materials. Impressive examples are the bisorbicillinoids, a family of fungal natural products thought to originate from the polyketide precursor sorbicillin. Utilizing the recombinant oxidoreductase SorbC from the sorbicillin biosynthetic gene cluster, a robust, fully stereoselective synthesis of bisorbicillinoid natural products and unnatural side‐chain analogues was developed.