Sequence-Specific Conformational Flexibility of SNARE Transmembrane Helices Probed by Hydrogen/Deuterium Exchange
01-Aug-2008
Biophysical Journal, 2008, 95 , 3, 1326 - 35 published on 01.08.2008
Biophysical Journal, online article
Biophysical Journal, online article
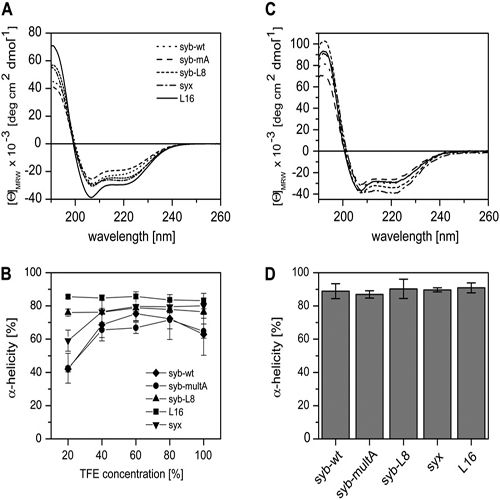
SNARE proteins mediate fusion of intracellular eukaryotic membranes and their -helical transmembrane domains are known to contribute to lipid bilayer mixing. Synthetic transmembrane domain peptides were previously shown to mimic the function of SNARE proteins in that they trigger liposome fusion in a sequence-specific fashion. Here, we performed a detailed investigation of the conformational dynamics of the transmembrane helices of the presynaptic SNAREs synaptobrevin II and syntaxin 1a. To this end, we recorded deuterium/hydrogen-exchange kinetics in isotropic solution as well as in the membrane-embedded state. In solution, the exchange kinetics of each peptide can be described by three different classes of amide deuteriums that exchange with different rate constants. These are likely to originate from exchange at different domains of the helices. Interestingly, the rate constants of each class vary with the TMD sequence. Thus, the exchange rate is position-specific and sequence-specific. Further, the rate constants correlate with the previously determined membrane fusogenicities. In membranes, exchange is retarded and a significant proportion of amide hydrogens are protected from exchange. We conclude that the conformational dynamics of SNARE TMD helices is mechanistically linked to their ability to drive lipid mixing.