Aligning, analyzing, and visualizing sequences for antibody engineering: Automated recognition of immunoglobulin variable region features
22-Oct-2016
PROTEINS, Pages 65-71, Volume85, Issue1, Pages 65-71, https://doi.org/10.1002/prot.25193
PROTEINS, online article
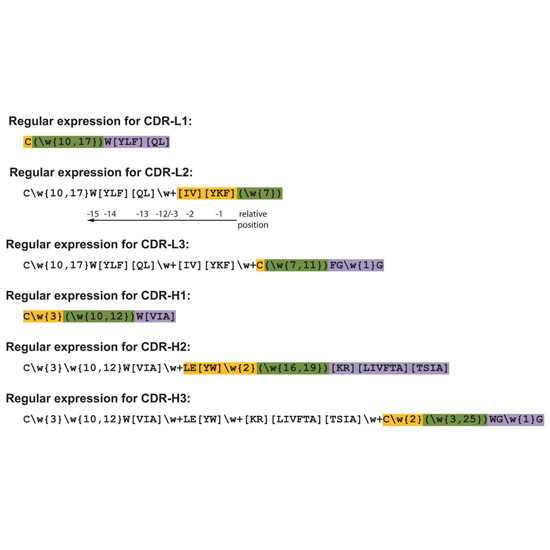
The analysis and comparison of large numbers of immunoglobulin (Ig) sequences that arise during an antibody selection campaign can be time‐consuming and tedious. Typically, the identification and annotation of framework as well as complementarity‐determining regions (CDRs) is based on multiple sequence alignments using standardized numbering schemes, which allow identification of equivalent residues among different family members but often necessitate expert knowledge and manual intervention. Moreover, due to the enormous length variability of some CDRs the benefit of conventional Ig numbering schemes is limited and the calculation of correct sequence alignments can become challenging. Whereas, in principle, a well established set of rules permits the assignment of CDRs from the amino acid sequence alone, no currently available sequence alignment editor provides an algorithm to annotate new Ig sequences accordingly. Here we present a unique pattern matching method implemented into our recently developed ANTICALIgN editor that automatically identifies all hypervariable and framework regions in experimentally elucidated antibody sequences using so‐called “regular expressions.” By combination of this widely supported software syntax with the unique capabilities of real‐time aligning, editing and analyzing extended sets of amino acid and/or nucleotide sequences simultaneously on a local workstation, ANTICALIgN provides a powerful utility for antibody engineering.