Self-priming determines high type I IFN production by plasmacytoid dendritic cells
16-Jan-2014
European Journal of Immunology, 2014, DOI: 10.1002/eji.201343806, Volume 44, Issue 3, pages 807–818 published on 16.01.2014
European Journal of Immunology, online article
European Journal of Immunology, online article
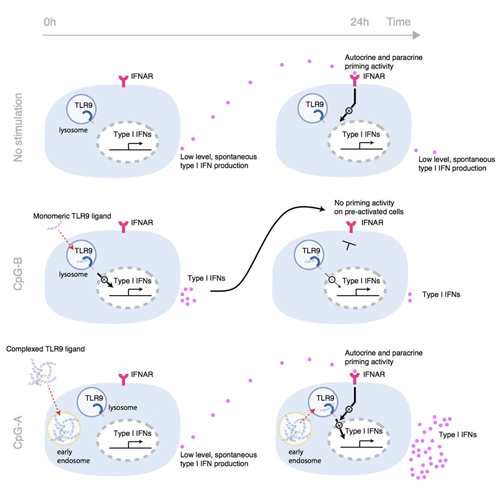
Plasmacytoid dendritic cells (pDCs) are responsible for the robust and immediate production of type I IFNs during viral infection. pDCs employ TLR7 and TLR9 to detect RNA and CpG motifs present in microbial genomes. CpG-A was the first synthetic stimulus available that induced large amounts of IFN-α (type I IFN) in pDCs. CpG-B, however, only weakly activates pDCs to produce IFN-α. Here, we demonstrate that differences in the kinetics of TLR9 activation in human pDCs are essential for the understanding of the functional difference between CpG-A and CpG-B. While CpG-B quickly induces IFN-α production in pDCs, CpG-A stimulation results in delayed yet maximal IFN-α induction. Constitutive production of low levels of type I IFN in pDCs, acting in a paracrine and autocrine fashion, turned out to be the key mechanism responsible for this phenomenon. At high cell density, pDC-derived, constitutive type I IFN production primes pDCs for maximal TLR responsiveness. This accounts for the high activity of higher structured TLR agonists that trigger type I IFN production in a delayed fashion. Altogether, these data demonstrate that high type I IFN production by pDCs cannot be simply ascribed to cell-autonomous mechanisms, yet critically depends on the local immune context.