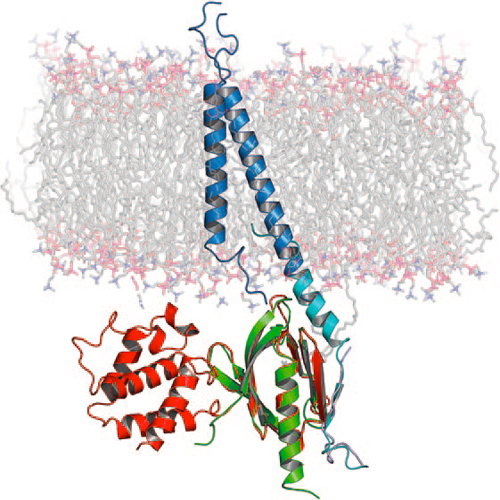
The Transmembrane Structure of Integrin aIIbb3: Significance for Signal Transduction
13-Jul-2009
Angewandte Chemie, 2010, 48 Issue 36, 6590-3 published on 13.07.2009
The formation of multicellular organisms requires concerted action by cells, which alter their adhesive and migratory behaviors. Cell adhesion and migration are tightly regulated by intra- and extracellular signals, which are conveyed through the cellular membrane by specialized receptors known as integrins. Integrins are the starting point of a variety of signaling cascades and are involved in a multitude of physiological events important to multicellular organism morphogenesis, ranging from cell adhesion to migration, apoptosis, and angiogenesis as well as pathophysiological behaviors such as those found in cancer metastasis. The transmembrane (TM) domains of integrins are at the center of integrin signaling. Recently, a structure of the TM domains of the aIIbb3 integrin has been reported that sheds light on the signal transduction mechanism of integrins. Integrins are essential TM proteins that couple the extracellular matrix to the cytoskeleton. They consist of noncovalently bound heterodimers, in which each subunit (a and b) contains one TM helix. With eighteen a and eight b subunit types identified, there are 24 known distinct heterodimer combinations with partially overlapping yet specific function.