The C-Terminal Regulatory Domain Is the RNA 5`-Triphosphate Sensor of RIG-I
01-Feb-2008
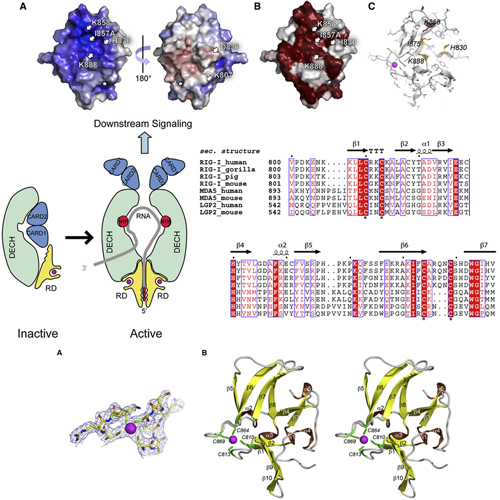
The ATPase RIG-I senses viral RNAs that contain 5`triphosphates in the cytoplasm. It initiates a signaling cascade that activates innate immune response by interferon and cytokine production, providing essential antiviral protection for the host. The mode of RNA 50-triphosphate sensing by RIG-I remains elusive. We show that the C-terminal regulatory domain RD of RIG-I binds viral RNA in a 50-triphosphate-dependent manner and activates the RIG-I ATPase by RNAdependent dimerization. The crystal structure of RD reveals a zinc-binding domain that is structurally related to GDP/GTP exchange factors of Rab-like GTPases. The zinc coordination site is essential for RIG-I signaling and is also conserved in MDA5 and LGP2, suggesting related RD domains in all three enzymes. Structure-guided mutagenesis identifies a positively charged groove as likely 5`-triphosphate-binding site of RIG-I. This groove is distinct in MDA5 and LGP2, raising the possibility that RD confers ligand specificity.