Substrate Transport Activation Is Mediated through Second Periplasmic Loop of Transmembrane Protein MalF in Maltose Transport Complex of Escherichia coli
26-Mar-2012
The Journal of Biological Chemistry, 2012, doi: 10.1074/jbc.M112.340679, 287, 17040-17049. published on 26.03.2012
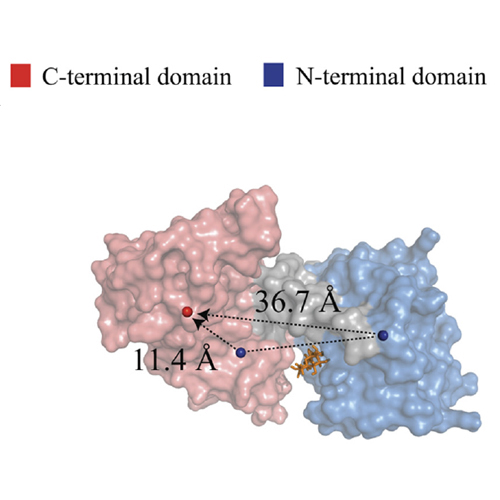
In a recent study we described the second periplasmic loop P2 of the transmembrane protein MalF (MalF-P2) of the maltose ATP-binding cassette transporter (MalFGK2-E) as an important element in the recognition of substrate by the maltose-binding protein MalE. In this study, we focus on MalE and find that MalE undergoes a structural rearrangement after addition of MalF-P2. Analysis of residual dipolar couplings (RDCs) shows that binding of MalF-P2 induces a semiopen state of MalE in the presence and absence of maltose, whereas maltose is retained in the binding pocket. These data are in agreement with paramagnetic relaxation enhancement experiments. After addition of MalF-P2, an increased solvent accessibility for residues in the vicinity of the maltose-binding site of MalE is observed. MalF-P2 is thus not only responsible for substrate recognition, but also directly involved in activation of substrate transport. The observation that substrate-bound and substrate-free MalE in the presence of MalF-P2 adopts a similar semiopen state hints at the origin of the futile ATP hydrolysis of MalFGK2-E.