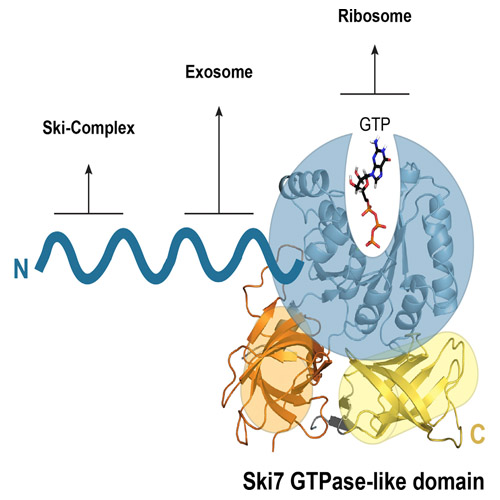
Saccharomyces cerevisiae Ski7 Is a GTP-Binding Protein Adopting the Characteristic Conformation of Active Translational GTPases
07-Jul-2015
Structure, Volume 23, Issue 7, p1336–1343, http://dx.doi.org/10.1016/j.str.2015.04.018
Ski7 is a cofactor of the cytoplasmic exosome in budding yeast, functioning in both mRNA turnover and non-stop decay (NSD), a surveillance pathway that degrades faulty mRNAs lacking a stop codon. The C-terminal region of Ski7 (Ski7C) shares overall sequence similarity with the translational GTPase (trGTPase) Hbs1, but whether Ski7 has retained the properties of a trGTPase is unclear. Here, we report the high-resolution structures of Ski7C bound to either intact guanosine triphosphate (GTP) or guanosine diphosphate-Pi. The individual domains of Ski7C adopt the conformation characteristic of active trGTPases. Furthermore, the nucleotide-binding site of Ski7C shares similar features compared with active trGTPases, notably the presence of a characteristic monovalent cation. However, a suboptimal polar residue at the putative catalytic site and an unusual polar residue that interacts with the γ-phosphate of GTP distinguish Ski7 from other trGTPases, suggesting it might function rather as a GTP-binding protein than as a GTP-hydrolyzing enzyme.