Probing Translation with Small-Molecule Inhibitors
25-Jun-2010
Cell, 2010, DOI 10.1016/j.chembiol.2010.06.003, Volume 17, Issue 6, 633-645 published on 25.06.2010
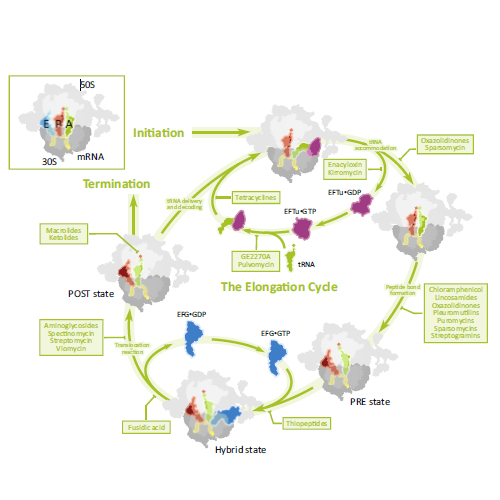
The translational apparatus of the bacterial cell remains one of the principal targets of antibiotics for the clinical treatment of infection worldwide. Since the introduction of specific translation inhibitors into clinical practice in the late 1940s, intense efforts have been made to understand their precise mechanisms of action. Such research has often revealed significant and sometimes unexpected insights into many fundamental aspects of the translation mechanism. Central to progress in this area, high-resolution crystal structures of the bacterial ribosome identifying the sites of antibiotic binding are now available, which, together with recent developments in single-molecule and fast-kinetic approaches, provide an integrated view of the dynamic translation process. Assays employing these approaches and focusing on specific steps of the overall translation process are amenable for drug screening. Such assays, coupled with structural studies, have the potential not only to accelerate the discovery of novel and effective antimicrobial agents, but also to refine our understanding of the mechanisms of translation. Antibiotics often stabilize specific functional states of the ribosome and therefore allow distinct translation steps to be dissected in molecular detail.