Inhibitors of glycosomal protein import provide new leads against trypanosomiasis
03-Jul-2017
Microbial Cell, Vol. 4, No. 7, pp. 229 - 232; DOI: 10.15698/mic2017.07.581
Microbial Cell, online article
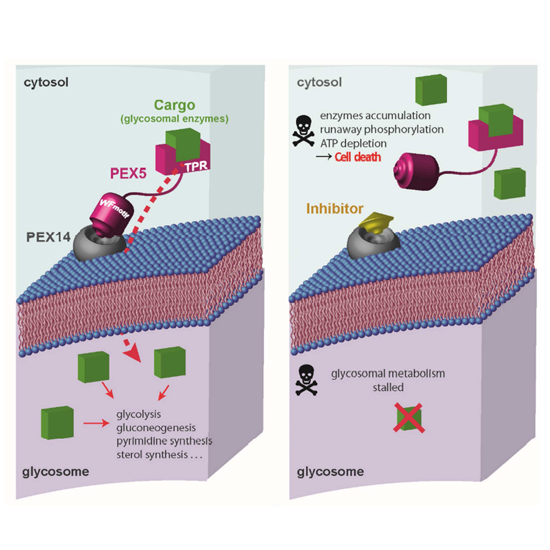
Vector-borne trypanosomatid parasite infections in tropical and sub-tropical countries constitute a major threat to humans and livestock. Trypanosoma brucei parasites are transmitted by tsetse fly and lead to African sleeping sickness in humans and Nagana in cattle. In Latin American countries, Trypanosoma cruzi infections spread by triatomine kissing bugs lead to Chagas disease. Various species of Leishmania transmitted to humans by phlebotomine sandflies manifest in a spectrum of diseases termed Leishmaniasis. 20 million people are currently infected with trypanosomatid parasites, leading to over 30,000 deaths annually and half billion people at risk of the infection. It is estimated that 300,000 Chagas infected people reside in the United States and 100,000 in Europe. Glycosomes are peroxisome-like organelles found only in trypanosomatids. Glycolysis occurs in the cytosol in all other organisms, but glycolytic enzymes and other metabolic pathways are compartmentalized inside glycosomes in trypanosomatids. Glycosomes are essential for the parasite survival and hence thought to be an attractive drug target. Our recent study [Dawidowski et al. Science (2017)] is the first to report small molecule inhibitors of glycosomal protein import. Using structure-based drug design, we developed small molecule inhibitors of the Trypanosoma PEX5-PEX14 protein-protein interaction that disrupt glycosomal protein import and kill the parasites. Oral treatment of T. brucei infected mice with PEX14 inhibitor significantly reduced the parasite levels with no adverse effect on mice. The study provides the grounds for further development of the glycosome inhibitors into clinical candidates and validates the parasite protein-protein interactions as drug targets.