Improving Oral Bioavailability of Peptides by Multiple N-Methylation: Somatostatin Analogues
26-Feb-2008
Angewandte Chemie International Edition, 2008, 47 (early view), 1-6 published on 26.02.2008
Angewandte Chemie, online article
Angewandte Chemie, online article
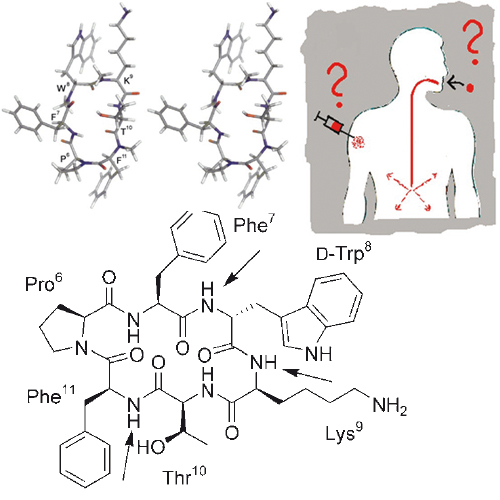
Full methyl jacket? A complete library of the N-methylated somatostatin cyclopeptidic analogue Veber–Hirschmann peptide cyclo(-PFwKTF-) is performed with the aim of improving its bioavailability. Several analogues from the library were found to bind to the somatostatin receptor in the nanomolar range and one of them shows a significant oral bioavailability of 10%. Conformational analysis shows that N-methylation is allowed at specific positions without affecting the bioactive conformation.