Improving oral bioavailability of cyclic peptides by N-methylation
31-Aug-2017
Bioorganic & Medicinal Chemistry, Volume 26, Issue 10, Pages 2766-2773, https://doi.org/10.1016/j.bmc.2017.08.031
Bioorganic & Medicinal Chemistry, online article
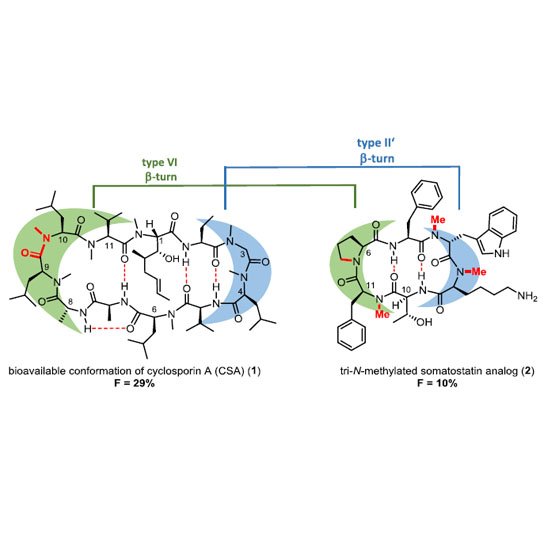
The renaissance of peptides in pharmaceutical industry results from their importance in many biological functions. However, low metabolic stability and the lack of oral availability of most peptides is a certain limitation. Whereas metabolic instability may be often overcome by development of small cyclic peptides containing d-amino acids, the very low oral availability of most peptides is a serious limitation for some medicinal applications. The situation is complicated because a twofold optimization – biological activity and oral availability – is required to overcome this problem. Moreover, most simple “rules” for achieving oral availability are not general and are applicable only to limited cases. Many structural modifications for increasing biological activities and metabolic stabilities of cyclic peptides have been described, of which N-alkylation is probably the most common. This mini-review focuses on the effects of N-methylation of cyclic peptides in strategies to optimize bioavailabilities.