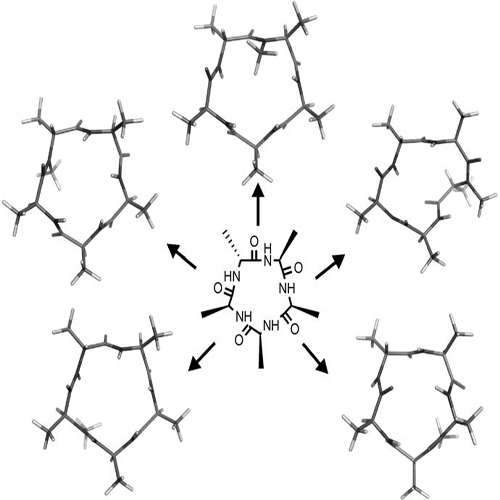
Conformational Preference and Potential Templates of N-Methylated Cyclic Pentaalanine Peptides
08-Feb-2008
Chemistry a European Journal, 2008, 14, 1508-17 published on 08.02.2008
Chemistry a European Journal, online article
Chemistry a European Journal, online article
Systematic N-methylation of all peptide bonds in the cyclic pentapeptide cyclo(-d-Ala-Ala4-) has been performed yielding 30 different N-methylated derivatives, of which only seven displayed a single conformation on the NMR time scale. The conformation of these differentially N-methylated peptides was recently reported by us (J. Am. Chem. Soc. 2006, 128, 15 164–15 172). Here we present the conformational characterization of nine additional N-methylated peptides from the previous library which are not homogeneous but exist as a mixture in which at least one conformation is preferred by over 80%. The structures of these peptides are investigated employing various 2D-NMR techniques, distance geometry calculations and further refined by molecular dynamics simulations in explicit DMSO. The comparison of the conformation of these nine peptides and the seven conformationally homogeneous peptides allow us to draw conclusions regarding the influence of N-methylation on the peptide backbone of cyclic pentapeptide of the class cyclo(-d-Ala-Ala4-). Here we present the different conformational classes of the peptides arising from the definitive pattern of N-methylation which can eventually serve as templates for the design of bioactive peptides.