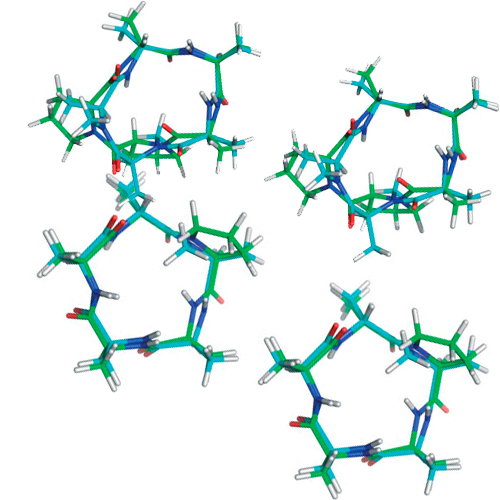
Can N-methylated amino acids serve as substitutes for prolines in conformational design of cyclic pentapeptides?
04-Nov-2008
Journal of Peptide Science, 2008, 15, 3, 141 - 46 published on 04.11.2008
Journal of Peptide Science online article
Journal of Peptide Science online article
The incorporation of proline into cyclic peptides seems to be the most promising way to induce -turn structures. Recently, however, it was shown that N-methylated amino acids might be even better suited than proline for introducing turn structures. Another property of proline, the ability to effect cis-peptide bonds, has also been reported for N-methylated amino acids. These findings raise the question if it might be possible to replace a proline by an N-methylated amino acid without altering the desired conformational features. The most important benefit of replacing proline by an N-methylated residue is that one recovers the side-chain functionalities, which could be used for enhancing binding selectivity, or to tune a cyclic peptide concerning its pharmacological properties. Here, we compare cyclic peptides containing one or two prolines or N-methylated alanines and a combination of both with respect to preferred conformations and cis-peptide bonds. In addition, the positions have been investigated where an N-alkylated amino acid has to be incorporated to mimic structural aspects usually introduced by proline residues.