ATP puts the brake on DNA double-strand break repair
11-Sep-2014
BioEssays, 2014, DOI: 10.1002/bies.201400102, Volume 36, Issue 12, pages 1170–1178, published on 11.09.2014
BioEssays, online article
BioEssays, online article
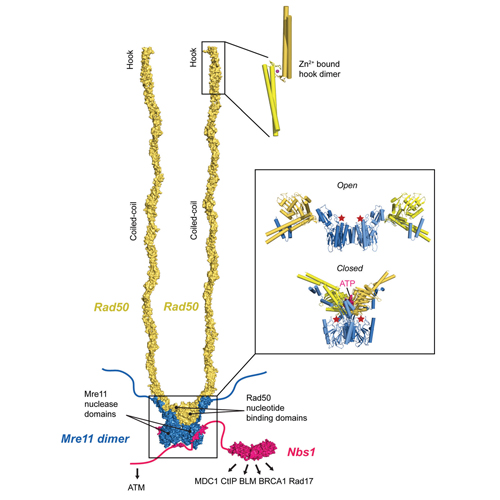
DNA double-strand breaks (DSBs) are one of the most deleterious forms of DNA damage and can result in cell inviability or chromosomal aberrations. The Mre11-Rad50-Nbs1 (MRN) ATPase-nuclease complex is a central player in the cellular response to DSBs and is implicated in the sensing and nucleolytic processing of DSBs, as well as in DSB signaling by activating the cell cycle checkpoint kinase ATM. ATP binding to Rad50 switches MRN from an open state with exposed Mre11 nuclease sites to a closed state with partially buried nuclease sites. The functional meaning of this switch remained unclear. A new study shows that ATP binding to Rad50 promotes DSB recognition, tethering, and ATM activation, while ATP hydrolysis opens the nuclease active sites to promote processing of DSBs. MRN thus emerges as functional switch that may coordinate the temporal transition from signaling to processing of DSBs.