A Cyclen-Based Tetraphosphinate Chelator for the Preparation of Radiolabeled Tetrameric Bioconjugates
23-Apr-2013
Chemistry - A European Journal, 2013, DOI: 10.1002/chem.201300338, Volume 19, Issue 24, pages 7748–7757, published on 23.04.2013
Chemistry - A European Journal, online article
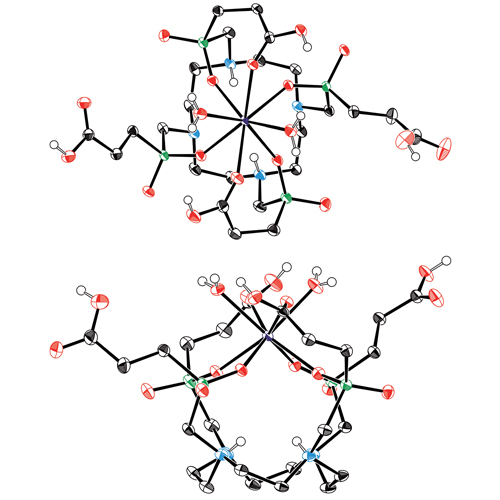
The cyclen-based tetraphosphinate chelator 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetrakis[methylene(2-carboxyethyl)phosphinic acid] (DOTPI) comprises four additional carboxylic acid moieties for bioconjugation. The thermodynamic stability constants (logKML) of metal complexes, as determined by potentiometry, were 23.11 for CuII, 20.0 for LuIII, 19.6 for YIII, and 21.0 for GdIII. DOTPI was functionalized with four cyclo(Arg-Gly-Asp-D-Phe-Lys) (RGD) peptides through polyethylene glycol (PEG4) linkers. The resulting tetrameric conjugate DOTPI(RGD)4 was radiolabeled with 177Lu and 64Cu and showed improved labeling efficiency compared with 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA). The labeled compounds were fully stable in transchelation challenges against trisodium diethylenetriaminepentaacetate (DTPA) and disodium ethylenediaminetetraacetic acid (ETDA), in phosphate buffered saline (PBS), and human plasma. Integrin αvβ3 affinities of the non-radioactive LuIII and CuII complexes of DOTPI(RGD)4 were 18 times higher (both IC50 about 70 picomolar) than that of the c(RGDfK) peptide (IC50=1.3 nanomolar). Facile access to tetrameric conjugates and the possibility of radiolabeling with therapeutic and diagnostic radionuclides render DOTPI suitable for application in peptide receptor radionuclide imaging (PRRI) and therapy (PRRT).