Total Chemical Synthesis of an Integral Membrane Enzyme: Diacylglycerol Kinase from Escherichia coli
18-Apr-2011
Angewandte Chemie, 2011, DOI: 10.1002/anie.201006686, Volume 50, Issue 17, pages 3988–3992, published on 18.04.2011
Angew. Chemie, online article
Angew. Chemie, online article
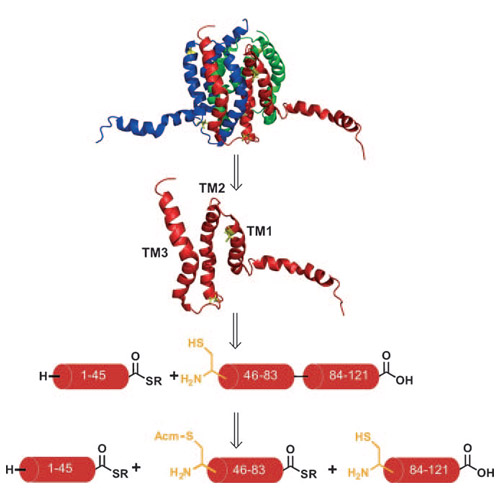
Recent progress in chemical protein synthesis has provided access to many small to medium-sized proteins. However, the highly important class of membrane proteins comprising multimembrane-spanning receptors and ion channels as well as integral membrane enzymes remains elusive with regard to chemical synthesis. Only certain moderately sized membrane proteins have been generated by chemical protein synthesis or semisynthesis. Synthesis of the constituent hydrophobic membrane-spanning peptide segments remains challenging owing to incomplete amino acid coupling steps and subsequent purification problems. Herein we report the chemical synthesis of Escherichia coli diacylglycerol kinase (DAGK), an integral membrane enzyme consisting of monomers with 121 amino acids each that form a homotrimer in a membrane environment as well as in detergent micelles. These homotrimers catalyze the conversion of diacylglycerol into phosphatidic acid and play a vital role in the lipid metabolism of Gram-negative bacteria, especially under conditions of environmental stress.