The chaperone Hsp90: changing partners for demanding clients
18-Mar-2013
Trends in Biochemical Sciences, 2013, doi:10.1016/j.tibs.2013.02.003, Volume 38, Issue 5, 253-262 published on 18.03.2013
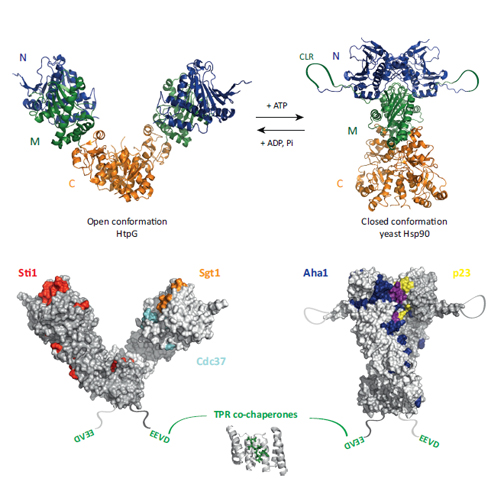
The heat shock protein (Hsp)90 chaperone machinery regulates the activity of hundreds of client proteins in the eukaryotic cytosol. It undergoes large conformational changes between states that are similar in energy. These transitions are rate-limiting for the ATPase cycle. It has become evident that several of the many Hsp90 co-chaperones affect the conformational equilibrium by stabilizing specific intermediate states. Consequently, there is an ordered progression of different co-chaperones during the conformational cycle. Asymmetric complexes containing two different co-chaperones may be important for the processing of the client protein, although our understanding of this aspect, as well as the details of the interaction of Hsp90 with client proteins, is still in its infancy.