Structural basis for coupling protein transport and N-glycosylation at the mammalian endoplasmic reticulum
13-Apr-2018
Science, 360, 215–219, DOI: 10.1126/science.aar7899
Science, online article
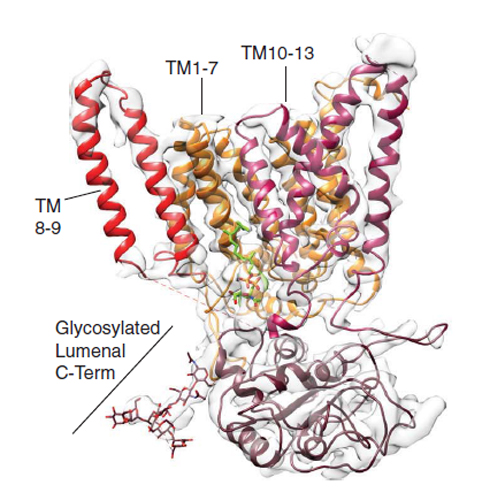
Protein synthesis, transport and N-glycosylation are coupled at the mammalian endoplasmic reticulum (ER) by complex formation between the ribosome, the Sec61 protein-conducting channel and the oligosaccharyltransferase (OST). Here, we used different cryo-electron microscopy approaches to determine structures of native and solubilized ribosome-Sec61-OST complexes. A molecular model for the catalytic OST subunit revealed how STT3A is integrated into the OST and how STT3 paralog specificity for translocon-associated OST is achieved. The OST subunit DC2 was placed at the interface between Sec61 and STT3A, where it acts as a versatile module for recruitment of STT3A-containing OST to the ribosome-Sec61 complex. This detailed structural view on the molecular architecture of the co-translational machinery for N-glycosylation provides the basis for a mechanistic understanding of glycoprotein biogenesis at the ER.