Structural basis for ArfA–RF2-mediated translation termination on mRNAs lacking stop codons
01-Dec-2016
Nature, doi:10.1038/nature20821
Nature, online article
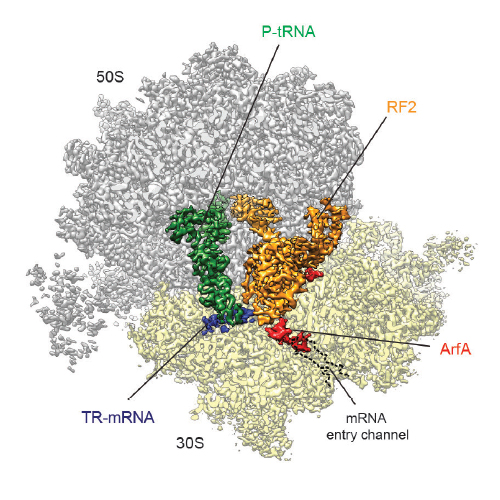
In bacteria, ribosomes stalled on truncated mRNAs lacking a stop codon are rescued by either the transfer-messenger RNA (tmRNA), alternative rescue factor A (ArfA) or ArfB rescue systems1. While tmRNA- and ArfB-ribosome structures have been elucidated2–7, structural insight into how ArfA recognizes the presence of truncated mRNAs and recruits the canonical termination release factor RF2 to rescue the stalled ribosomes is lacking. Here we present a cryo-electron microscopy reconstruction of a ribosome stalled on a truncated mRNA in the presence of ArfA and RF2. The structure reveals that the C-terminus of ArfA binds within the mRNA entry channel on the small ribosomal subunit, explaining how ArfA distinguishes ribosomes bearing truncated versus full-length mRNAs. The N-terminal region of ArfA establishes multiple interactions with the decoding domain of RF2, illustrating how ArfA recruits RF2 to the stalled ribosome. Additionally, ArfA is observed to stabilize a unique conformation of the switch loop of RF2, which mimics the canonical translation termination state by directing the catalytically important GGQ motif within domain 3 of RF2 towards the peptidyl-transferase center (PTC) of the ribosome. Thus, our structure reveals not only how ArfA recruits RF2 to the ribosome but also how it promotes an active conformation of RF2 to enable translation termination in the absence of a stop codon.