Proximity‐Triggered Covalent Stabilization of Low‐Affinity Protein Complexes In Vitro and In Vivo
28-Sep-2017
Angew. Chem. Int. Ed. 2017, 56, 15737 –15741, https://doi.org/10.1002/anie.201706927
Angew. Chem. Int. Ed., online article
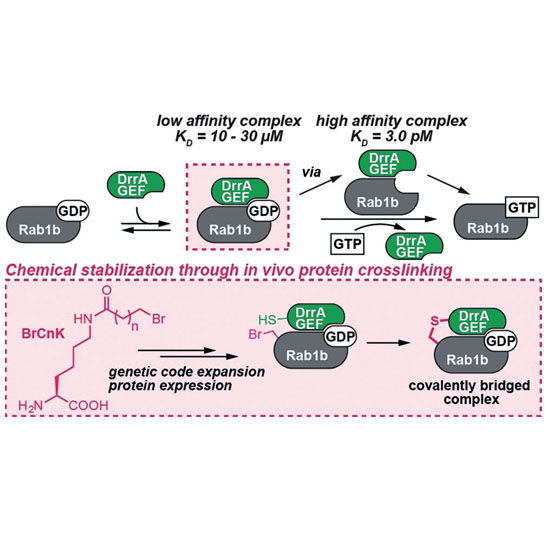
The characterization of low‐affinity protein complexes is challenging due to their dynamic nature. Here, we present a method to stabilize transient protein complexes in vivo by generating a covalent and conformationally flexible bridge between the interaction partners. A highly active pyrrolysyl tRNA synthetase mutant directs the incorporation of unnatural amino acids bearing bromoalkyl moieties (BrCnK) into proteins. We demonstrate for the first time that low‐affinity protein complexes between BrCnK‐containing proteins and their binding partners can be stabilized in vivo in bacterial and mammalian cells. Using this approach, we determined the crystal structure of a transient GDP‐bound complex between a small G‐protein and its nucleotide exchange factor. We envision that this approach will prove valuable as a general tool for validating and characterizing protein–protein interactions in vitro and in vivo.