IspC as Target for Antiinfective Drug Discovery: Synthesis, Enantiomeric Separation, and Structural Biology of Fosmidomycin Thia Isosters
13-Sep-2013
J. Med. Chem., 2013, DOI: 10.1021/jm4012559, 56 (20), pp 8151–8162 published on 13.09.2013
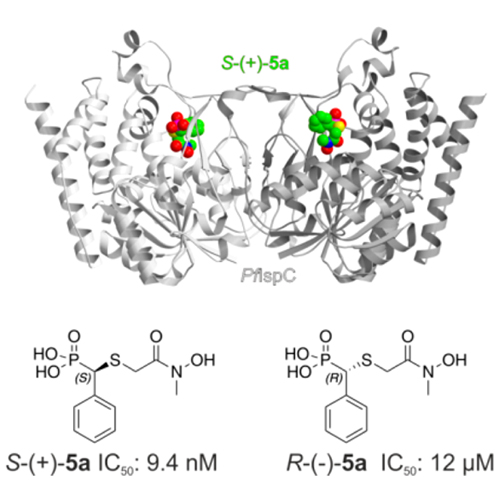
The emergence and spread of multidrug-resistant pathogens are widely believed to endanger human health. New drug targets and lead compounds exempt from cross-resistance with existing drugs are urgently needed. We report on the synthesis and properties of “reverse” thia analogs of fosmidomycin, which inhibit the first committed enzyme of a metabolic pathway that is essential for the causative agents of tuberculosis and malaria but is absent in the human host. Notably, IspC displays a high level of enantioselectivity for an α-substituted fosmidomycin derivative.