How antibodies fold
22-Dec-2009
Trends in Biochemical Sciences, 2009, doi:10.1016/j.tibs.2009.11.005, Volume 35, Issue 4, 189-198 published on 22.12.2009
Trends in Biochemical Sciences, online article
Trends in Biochemical Sciences, online article
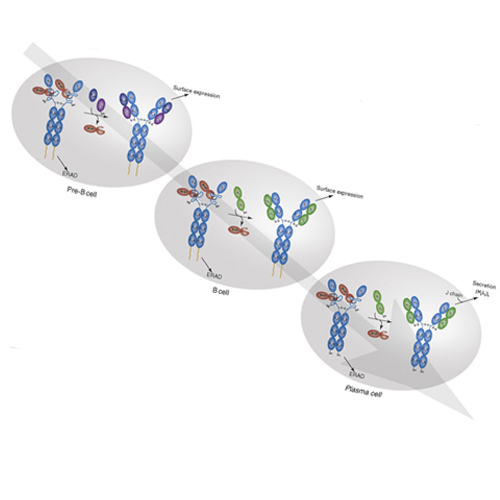
B cells use unconventional strategies for the production of a seemingly unlimited number of antibodies from a very limited amount of DNA. These methods dramatically increase the likelihood of producing proteins that cannot fold or assemble appropriately. B cells are therefore particularly dependent on ‘quality control’ mechanisms to oversee antibody production. Recent in vitro experiments demonstrate that Ig domains have evolved diverse folding strategies ranging from robust spontaneous folding to intrinsically disordered domains that require assembly with their partner domains to fold; in vivo experiments reveal that these different folding characteristics form the basis for cellular checkpoints in Ig transport. Taken together, these reports provide a detailed understanding of how B cells monitor and ensure the functional fidelity of Ig proteins.