Transferring the entatic-state principle to copper photochemistry
15-Jan-2018
Nature Chemistry, doi:10.1038/nchem.2916
Nature Chemitry, online article
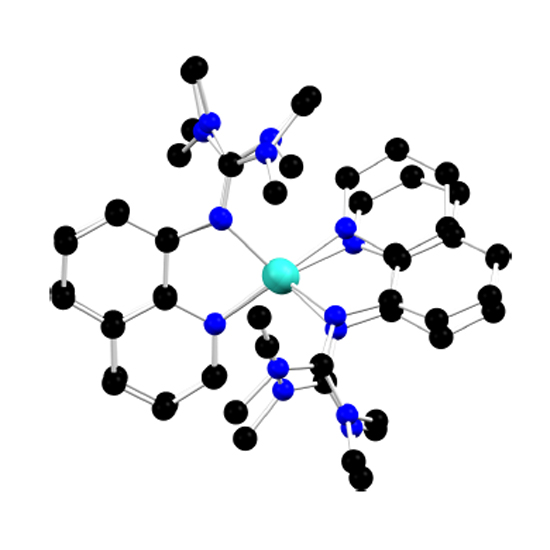
The entatic state denotes a distorted coordination geometry of a complex from its typical arrangement that generates an improvement to its function. The entatic-state principle has been observed to apply to copper electron-transfer proteins and it results in a lowering of the reorganization energy of the electron-transfer process. It is thus crucial for a multitude of biochemical processes, but its importance to photoactive complexes is unexplored. Here we study a copper complex—with a specifically designed constraining ligand geometry—that exhibits metal-to-ligand charge-transfer state lifetimes that are very short. The guanidine–quinoline ligand used here acts on the bis(chelated) copper(I) centre, allowing only small structural changes after photoexcitation that result in very fast structural dynamics. The data were collected using a multimethod approach that featured time-resolved ultraviolet–visible, infrared and X-ray absorption and optical emission spectroscopy. Through supporting density functional calculations, we deliver a detailed picture of the structural dynamics in the picosecond-to-nanosecond time range.