The Hammett Relationship and Reactions in the Excited Electronic State Hemithioindigo Z/E-Photoisomerization
05-Jan-2008
The Journal of Physical Chemistry, 2008, 112, 4, 581- 88 published on 05.01.2008
The Journal of Physical Chemistry , online article
The Journal of Physical Chemistry , online article
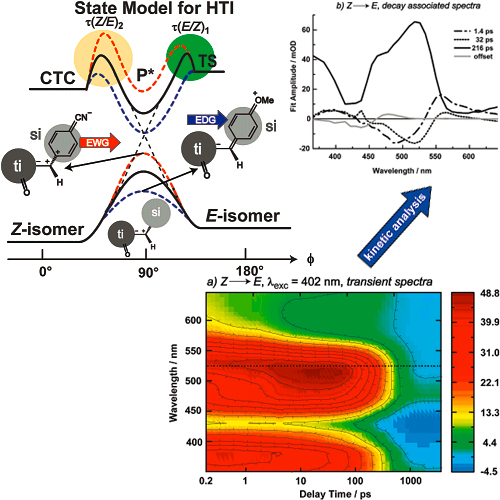
The photochemical reaction dynamics of a set of photochromic compounds based on thioindigo and stilbene molecular parts (hemithioindigos, HTI) are presented. Photochemical Z/E isomerization around the central double bond occurs with time constants of 216 ps (Z → E) and 10 ps (E → Z) for a 5-methyl-hemithioindigo. Chemical substitution on the stilbene moiety causes unusually strong changes in the reaction rate. Electron-donating substituents in the position para to the central double bond (e.g., para-methoxy) strongly accelerate the reaction, while the reaction is drastically slowed by electron-withdrawing groups in this position (e.g., para-nitrile). We correlate the experimental data of seven HTI-compounds in a quantitative manner using the Hammett equation and present a qualitative explanation for the application of ground-state Hammett constants to describe the photoisomerization reaction.