Single Molecule Fluorescence of Native and Refolded Peridinin–Chlorophyll–Protein Complexes
17-Jan-2008
Journal of Fluorescence, 2008, 18, 611-7 published on 17.01.2008
Journal of Fluorescence, online article
Journal of Fluorescence, online article
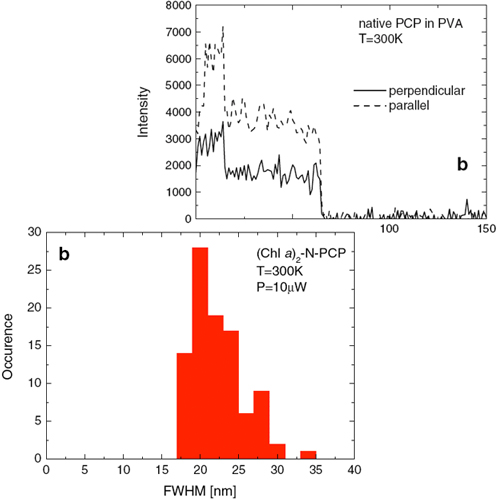
Single molecule spectroscopy was applied to study the optical properties of native and refolded peridinin–chlorophyll–protein (PCP) complexes. The native system is a trimer with six chlorophyll a (Chl a) molecules, while the refolded one contains two Chl a and resembles structurally and spectroscopically the PCP monomer. The fluorescence emission of single PCP complexes strongly broadens with increasing excitation power. Simultaneously, the distribution of fluorescence maximum frequencies is also broadened. These spectral changes are attributed to photoinduced conformational changes of the protein that influence the fluorescence of embedded chromophores. Comparison of fluorescence intensities measured for PCP complexes in two different solvents indicates that the native PCP trimers are preserved in EDTA Tris buffer, while in PVA polymer matrix only monomers are stable.