Dynamics of heat shock protein 90 C-terminal dimerization is an important part of its conformational cycle
24-Aug-2010
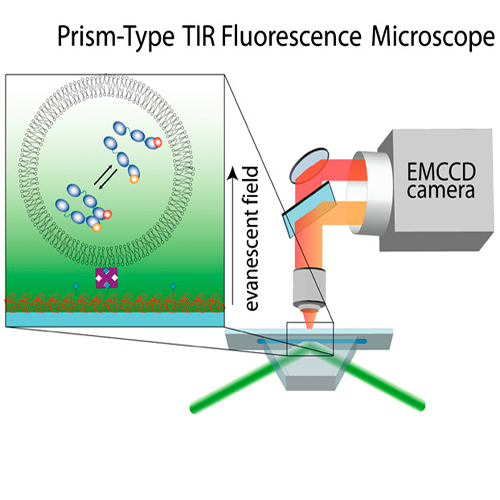
The molecular chaperone heat shock protein 90 (Hsp90) is an important and abundant protein in eukaryotic cells, essential for the activation of a large set of signal transduction and regulatory proteins. During the functional cycle, the Hsp90 dimer performs large conformational rearrangements. The transient N-terminal dimerization of Hsp90 has been extensively investigated, under the assumption that the C-terminal interface is stably dimerized. Using a fluorescence-based single molecule assay and Hsp90 dimers caged in lipid vesicles, we were able to separately observe and kinetically analyze N- and C-terminal dimerizations. Surprisingly, the C-terminal dimer opens and closes with fast kinetics. The occupancy of the unexpected C-terminal open conformation can be modulated by nucleotides bound to the N-terminal domain and by N-terminal deletion mutations, clearly showing a communication between the two terminal domains. Moreover our findings suggest that the C- and N-terminal dimerizations are anticorrelated. This changes our view on the conformational cycle of Hsp90 and shows the interaction of two dimerization domains.