Direct Observation of Active Protein Folding Using Lock-in Force Spectroscopy
17-Aug-2007
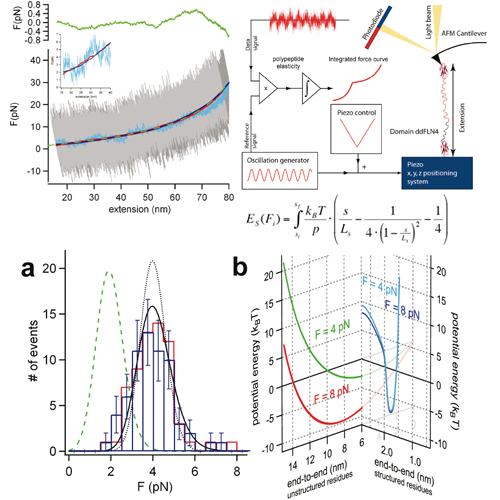
Direct observation of the folding of a single polypeptide chain can provide important information about the thermodynamic states populated along its folding pathway. In this study we present a lock-in force spectroscopy technique that improves resolution of AFM force spectroscopy to 400 fN. Using this technique we show that immunoglobulin domain 4 from dictyostelium discoideum filamin (ddFLN4) refolds against forces of approx. 4 pN. Our data show folding of this domain proceeds directly from an extended state and no thermodynamically distinct collapsed state of the polypeptide prior to folding is populated. Folding of ddFLN4 under load proceeds via an intermediate state. Three-state folding allows ddFLN4 to fold against significantly larger forces than would be possible for a mere two-state folder. We present a general model for protein folding kinetics under load that can predict refolding forces based on chain length and zero force refolding rate.