Chaperone Action at the Single-Molecule Level
03-Sep-2014
Chem. Rev., 2014, DOI: 10.1021/cr400326k, 114 (1), pp 660–667 published on 03.09.2014
Chem. Rev., online article
Chem. Rev., online article
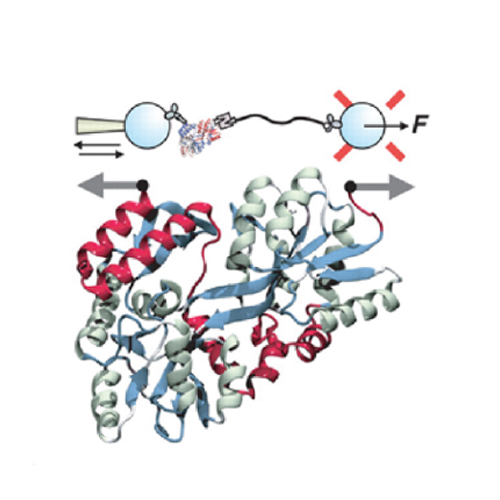
INTRODUCTION: Natively folded proteins generally have a significant number of hydrophobic residues that cluster together to form a hydrophobic core. However, during the vectorial synthesis on the ribosome and subsequent folding, these hydrophobic residues are exposed. Because folding occurs in a highly crowded environment, exposed residues can lead to undesired interactions that irreversibly harm the folding process. In particular, they can result in the formation of misfolded states and aggregation.