actinin-titin-interaction-a-dynamic-and-mechanically-stable-cluster-of-bonds-in-the-muscle-z-disk
17-Jan-2017
PNAS, vol. 114, no. 5, 1015–1020, https://doi.org/10.1073/pnas.1612681114
PNAS, online article
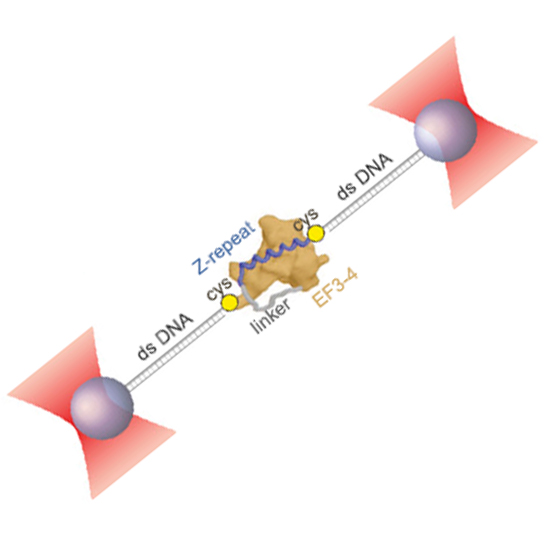
Stable anchoring of titin within the muscle Z-disk is essential for preserving muscle integrity during passive stretching. One of the main candidates for anchoring titin in the Z-disk is the actin cross-linker α-actinin. The calmodulin-like domain of α-actinin binds to the Z-repeats of titin. However, the mechanical and kinetic properties of this important interaction are still unknown. Here, we use a dual-beam optical tweezers assay to study the mechanics of this interaction at the single-molecule level. A single interaction of α-actinin and titin turns out to be surprisingly weak if force is applied. Depending on the direction of force application, the unbinding forces can more than triple. Our results suggest a model where multiple α-actinin/Z-repeat interactions cooperate to ensure long-term stable titin anchoring while allowing the individual components to exchange dynamically.